Acute lymphoblastic leukemia (ALL) comprises hematological malignancies affecting B or T cell precursors (BCP-ALL or T-ALL). Despite considerable progress made over the past three decades in the treatment of childhood ALL [5-year disease survival rate (5-y DFS): 90%], the prognosis of adult ALL remains relatively poor (5-y DFS: 50-70%). Hence, gaining a deeper understanding of the pathogenic factors is of timely importance. Recent advancements in next-generation genome sequencing technologies have facilitated the refinement of disease diagnosis and prognosis. However, due to the inherent genetic diversity among patients, additional tools are needed to unravel the complex etiological basis and improve risk stratification for personalized therapeutic interventions, particularly in adult ALL. Metabolomics offers a powerful approach that enables the comprehensive exploration of cellular states by analyzing metabolites quantitatively and qualitatively, which reflects altered enzyme kinetic activities and changes in metabolic reactions. It was previously reported that most bone marrow metabolites could be detected in plasma in BCP-ALL (81%). In this study, we reported an integrated analysis of serum metabolites using liquid chromatography/mass spectrometry as well as the metabolic gene expression profiles from RNA-sequencing data of bone marrow blasts, generating a comprehensive metabolic information dataset (CMID) in 211 adult ALL patients (BCP-ALL: n=160; T-ALL: n=51) who participated in our recent clinical trial (ChiCTR-ONRC-14004968).
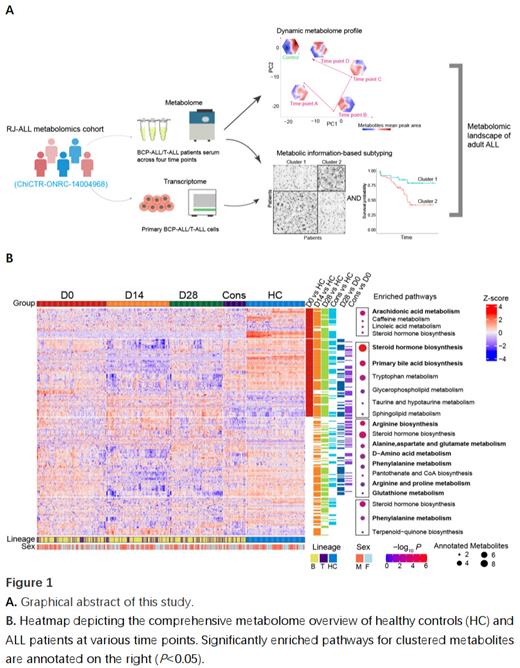
The graphical abstract is depicted in Figure 1A. Serum metabolomic landscape of adult ALL was obtained at various time points during treatment, including samples at diagnosis (Day 0, D0, n=198), during the induction phase (D14, n=177 and D28, n=147) and the early consolidation phase (Cons, n=58). These samples were subject to comparison to those from 164 age/sex-matched healthy controls (HC). Using CMID, we further subtyped BCP-ALL (clusters BC1 and BC2, and a special cluster of Ph + ALL) and T-ALL (clusters TC1 and TC2), revealing that BC2 and TC1 were associated with relatively favorable outcome, compared respectively to BC1 (OS: P<0.05) and TC2 (OS: P<0.05). Regarding serum metabolomic profiles over the treatment course, we found a number of featured disturbances ( Figure 1B). Aberrant lipid-related metabolisms, such as steroid metabolism, were detectable throughout different phases. In contrast, the metabolism of amino acids and a number of metabolites pumped by ATP-binding cassette transporter were severely impacted by therapeutic agents during the induction phase (on D14). Notably, at the time of diagnosis, primary bile acid biosynthesis was enriched, consistent with a previous study on pediatric ALL. In our functional experiment, inhibiting the synthesis of bile acid precursors such as cholesterol with Simvastatin suppressed leukemic cell viability in 10 ALL cell lines, indicating a potential role of this pathway in leukemogenesis. In addition, we identified arachidonic acid (AA) metabolism enrichment in the favorable BC2 subgroup. Coincidentally, glycerophospholipid metabolism, which provides substrates for AA synthesis, was also enriched in the TC1 subgroup, together with an active AA metabolism. Increasing data suggests that AA metabolites act as immunoregulators in cancer, thus we hypothesize that they could play a role in ALL prognosis by regulating the immune reaction against leukemic cells in the hematopoietic microenvironment.
In our further efforts to address the correlation between CMID-based subtypes and known genetic events in Ph --BCP-ALL and T-ALL leukemogenesis, we discovered that some favorable genetic markers (ZNF-384/362 fusions and high ploidy) tended to fall into BC2, while NRAS mutations were only observed in TC1. On the other hand, both CMID-based subtypes and genetic events-based ones stand as independent factors in multivariate Cox analysis for risk stratification of BCP-ALL, demonstrating an added value of the CMID subtypes in the prognosis of adult BCP-ALL. Our work has thus highlighted the significance of altered metabolome in ALL pathogenesis, proved its clinical relevance for prognosis stratification, and identified its potential vulnerabilities for improving tailored therapy.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal